JGIM: CTeL Research Finds Telehealth Payment Parity Reduces Overdose Risk | Supports Need for DEA to Finalize Special Registration Before EOY Waiver Expiration
CTeL’s new study published last week in the Journal of General Internal Medicine compares overdose rates in states that implemented telehealth payment parity laws with those that did not.
On December 31, 2025, DEA waivers allowing for the virtual prescribing of controlled substances by telehealth will end unless DEA provides an extension to these waivers. Absent action from the agency, clinicians will no longer be able to prescribe controlled medications via telemedicine for patients who have not had an in-person visit. This week’s Health inTEL blog will look at the legal and regulatory history of this issue and highlight CTeL’s newly-published research in the Journal of General Internal Medicine.
The Ryan Haight Online Pharmacy Consumer Protection Act of 2008 established the legal framework for prescribing controlled medications via telemedicine. To meet the requirements of Ryan Haight Act, the patient receiving a telemedicine prescription for a controlled medication needs to have either: a) An in-person medical evaluation with the prescriber, or (b) Be treated at a DEA-registered hospital or clinic under 21 U.S.C. § 823(f) to engage with the prescriber.
In response to the COVID-19 pandemic shuttering many in-person medical visits, the DEA issued a waiver to this requirement allowing clinicians to prescribe these medications without an initial in-person visit. Following the expiration of the COVID-19 public health emergency (PHE) declaration in 2023, the DEA has issued three temporary extensions of this waiver – now set to expire on December 31.
The Ryan Haight Act also issued a directive to the DEA: establish a regulatory framework for a ‘special registration’ for clinicians prescribing controlled medications via telemedicine. Congress renewed this directive in 2018 with the passage of the SUPPORT Act. Following expiration of the COVID-19 PHE, the DEA proposed two separate rules to establish a special registration, with listening sessions in between. The Agency also issued two final rules set to take effect in 2026 – one concerning telemedicine prescribing of buprenorphine and another for Veterans Affairs clinicians.
Last week, CTeL held a closed-door briefing for Congressional staff on this issue. We highlighted the potential impacts of these waivers expiring without a final rule, including:
Barriers to accessing care, especially in rural areas,
Increased ER visits due to less access to specialty care,
Interrupted treatments for patients relying on telehealth,
Increased risk of self-medication without oversight,
Worsened mental health / substance use outcomes,
Loss of provider-patient continuity,
Administrative burden on providers,
Legal and regulatory confusion,
Patient abandonment, and
Companies going out of business.
Recognizing these impacts, CTeL has been an active voice in highlighting the need for long-term policy action from the DEA to ensure regulatory stability and patient continuity of care. Earlier this month, CTeL sent a letter to the new DEA Administrator Terry Cole, renewing our call for action.
As a research institute, CTeL recognizes the importance of evidence-based policymaking. In a meeting with DEA officials, agency leaders emphasized the relative lack of robust research on the relationship between telehealth access, drug diversion, and overdose rates. A long-standing concern from the DEA is the proliferation of controlled substances, as the agency is tasked with enforcing federal laws on their manufacture and distribution. The growth of online direct-to-consumer (DTC) telehealth platforms designed around prescribing medications, particularly controlled medications, has caused concern within the agency. This concern intensified after a number of high-profile indictments last year brought against DTC telehealth companies regarding their prescribing practices.
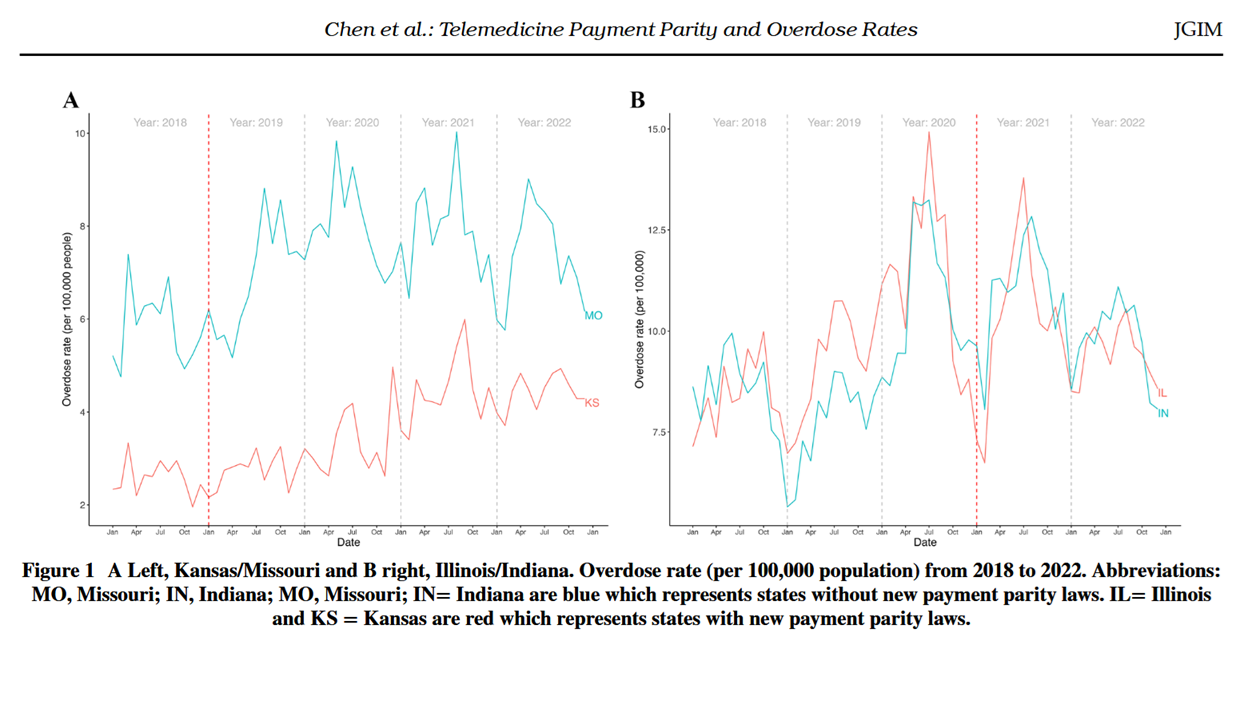
Recognizing this, CTeL undertook a study with our Research Fellows and CTeL partner Charm Economics to further explore the relationship between telehealth access and overdose rates. Performing a difference-in-difference analysis, our research team compared two pairs of states: Kansas/Missouri and Illinois/Indiana. These states were chosen for their relative similarity aside from one key difference: one state expanded telehealth access through payment parity laws, and the other did not. Our team found that in both state pairs, the state that had implemented telehealth parity laws saw a statistically significant decrease in overdose rates.
We are excited to announce that these findings have been published in the Journal of General Internal Medicine, under the title Concise Research Report: Telemedicine Payment Parity and Overdose Rates. Our study joins a growing body of literature demonstrating the benefits of telemedicine prescribing, particularly for the treatment of substance use disorder. This publication could not come at a more critical time – as we approach the expiration of the DEA’s telemedicine prescribing waivers, we again urge swift and long-term action from the Agency.